- Review
- Open access
- Published:
COVID-19 and lipids. The role of lipid disorders and statin use in the prognosis of patients with SARS-CoV-2 infection
Lipids in Health and Disease volume 20, Article number: 141 (2021)
Abstract
The global coronavirus disease 2019 (COVID-19) pandemic caused by the SARS-CoV-2 coronavirus started in March 2020. The conclusions from numerous studies indicate that people with comorbidities, such as arterial hypertension, diabetes, obesity, underlying cardiovascular disease, are particularly vulnerable to the severe course of COVID-19. The available data also suggest that patients with dyslipidemia, the most common risk factor of cardiovascular diseases, are also at greater risk of severe course of COVID-19. On the other hand, it has been shown that COVID-19 infection has an influence on lipid profile leading to dyslipidemia, which might require appropriate treatment. Owing to antiviral, anti-inflammatory, immunomodulatory, and cardioprotective activity, statin therapy has been considered as valuable tool to improve COVID-19 outcomes. Numerous observational studies have shown potential beneficial effects of lipid-lowering treatment on the course of COVID-19 with significant improved prognosis and reduced mortality.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first identified in December 2019 in Wuhan, China. As a coronavirus belonging to the family of zoonotic viruses, its genetic material is a single-stranded ribonucleic acid. SARS-Cov-2 causes the Coronavirus Disease 2019 (COVID-19), an acute infectious disease of the respiratory system, which was declared a global pandemic by the World Health Organization on March 11, 2020. COVID-19 is characterized by a mortality of about 2,3% and a transmission rate of about 2.5–3.0 (the number of newly infected people per previously infected person) [1]. Over 4.4 million people worldwide have died from COVID-19 so far (September 2021).
In the course of COVID-19, there are three phases which differ in the severity of the course, covering the time from early infection to recovery or death [2]. From a clinical point of view, the symptoms occurring after contracting COVID-19 are important. A meta-analysis of 15 studies by Lopez-Leon et al. assessed the incidence of long-term effects of COVID-19, wchich included: fatigue (58%), headache (44%), attention disorders (27%), hair loss (25%) and dyspnea (24%) [3]. Based on a meta-analysis of 38 studies conducted by Iqbal et al. it was found that fatigue and dyspnoea were the most prevalent symptoms in acute post-COVID-19 and fatigue and sleep disturbance in chronic post-COVID-19 syndrome [4].
A recently published meta-analysis of 27 studies by Shi et al. summarized our understanding of the factors that predict COVID-19 mortality. The most important predictors of death in the course of COVID-19 include: renal replacement therapy (relative risk: RR = 53.5; 95%CI: 22.4–127.3; p < 0.001), invasive mechanical ventilation (RR = 29.3; 95% CI: 21.5–39.9; p < 0.001), high level of procalcitonin (RR = 19.9; 95% CI: 7.31–53.9; p < 0.001), chronic kidney disease (RR = 8.37; 95%CI: 3.94–17.8; p < 0.001) and cerebrovascular diseases (RR = 7.66; 95% CI: 3.87–15.2; p < 0.001) [5]. The impact of cardiovascular diseases (CVD) on the prognosis of COVID-19 patients was assessed in a meta-analysis of 51 studies by Bae et al. It was shown that factors deteriorating the prognosis of COVID-19 (severe course or death) included: arterial hypertension (odds ratio: OR = 2.50; 95% CI: 2.15–2.90), diabetes (OR = 2.25; 95% CI: 1.89–2.69) and other CVD (OR = 3.11; 95% CI: 2.55–3.79) [6]. The results of the above studies indicate that CVD significantly worsens the prognosis of patients with COVID-19 [7]..
Taking into account the high prevalence of lipid disorders and their important role in shaping cardiovascular risk [8], a literature review was carried out on lipidological problems and COVID-19. Based on a review of the latest literature, the article describes a potential relationship between the incidence of lipid disorders and the prognosis of COVID-19 patients, the impact of SARS-CoV-2 infection on lipid metabolism and the role of statins in the treatment of COVID-19 patients. Some available literature data has been critically discussed and clinical recommendations regarding statin use during the COVID-19 pandemic have been provided. This article briefly and comprehensively presents the latest findings on lipidology and COVID-19. Moreover, the article also summarizes the latest guidelines for the use of statins in COVID-19.
Influence of dyslipidemia on the course and prognosis in COVID-19 patients
Cholesterol plays an important role in SARS-CoV-2 entry into host cells [9]. In an in vitro study, the depletion of membrane-bound cholesterol in ACE2 (angiotensin converting enzyme-2)-expressing cells led to a reduced infectivity of SARS-CoV, since the binding of the spike protein was reduced by 50% [10]. In patients with dyslipidemia, the systemic cholesterol content is increased, which may lead to an increase in the number of ACE2 receptors in the lipid rafts of cells and facilitate the penetration of SARS-CoV-2 into them. The role of lipoproteins and their receptors in the penetration of SARS-CoV-2 into cells requires further biochemical research [9]. It has been suggested that lipids, including fatty acids, interact with SARS-CoV-2 and may be a potential intervention strategy against COVID-19 [11]. Moreover, it is indicated that cholesterol, by influencing SARS-CoV-2’s S spike configuration, may increase the affinity for ACE2 and thus the infectivity of this coronavirus [12]. An important role for the scavenger receptor, class B type 1 (SR-B1) in penetration of SARS-CoV-2 into the host cell has also been suggested. In experimental studies, it was found that the use of the SR-B1 antagonist reduced the infectivity of SARS-CoV-2 [13].
The relationship between lipid disorders, their treatment and the severity of COVID-19 has been an object of interest in some recently published observational studies, which indicate that dyslipidemia is associated with a poorer prognosis in COVID-19 patients. In a meta-analysis of 9 observational studies conducted by Atmosudigdo et al. which included 3663 patients with COVID-19, dyslipidemia occurred in 18% of patients. It was shown that dyslipidemia was associated with a 39% increase in the risk of mortality in COVID-19 patients (RR = 1.39; 95% CI: 1.02–1.88; p = 0.010). Moreover, dyslipidemia also increased the risk of severe course of COVID-19 by 39% (RR = 1.39; 95% CI: 1.03–1.87; p = 0.008). In the meta-regression analysis, it was found that such an adverse effect of dyslipidemia on the course and prognosis of COVID-19 was more pronounced in older men with concomitant arterial hypertension. The authors of this meta-analysis indicate that dyslipidemia may potentially be a factor that worsens the course and prognosis of COVID-19. However, there were lacking data concerning comorbidities and medications taken by patients [14]. Another meta-analysis of 7 studies by Hariyanto and Kurniawan including 6922 patients with COVID-19 showed similar association between dyslipidemia and the course of COVID-19. The authors proved that dyslipidemia can possibly increase the risk of severe COVID-19 by 39% (RR = 1.39; 95% CI: 1.03–1.87; p = 0.03) [15]. Likewise, Santos et al. in a study involving 3711 patients with COVID-19, showed that dyslipidemia was associated with an increased risk of a severe course of COVID-19 (OR = 12; 95% CI: 1.33–108; p = 0.03) [16]. This was also supported by Choi et al. in the meta-analysis which showed that patients with COVID-19 and dyslipidemia were characterized by 49% (RR = 1.49; 95% CI: 1.11–2.01; p = 0.01) higher risk of severe COVID-19 than those with normal lipid profile. On the other hand, it was shown that the increased concentration of total cholesterol, low density lipoprotein (LDL), high density lipoprotein (HDL) and triglycerides in the serum was inversely correlated with the severity of COVID-19 [17]. A recently published meta-analysis of 28 studies by Liu et al. summarized the existing data on the impact of dyslipidemia on the prognosis of patients with COVID-19. The study included 12,995 patients with COVID-19. Dyslipidemia has been shown to increase the risk of severe course of COVID-19 (OR = 1.27, 95%CI: 1.11–1.44, p = 0.038) and the risk of death due to COVID-19 (OR = 2.13, 95%CI: 1.84–2.47, p = 0.001). The researchers conclude that the occurrence of dyslipidemia in patients with COVID-19 may worsen their prognosis [18].
A study by Masana et al., involving 1411 patients with COVID-19, assessed the usefulness of serum total cholesterol, LDL, non-HDL, HDL cholesterol and triglicerydes to predict the COVID-19 prognosis (severe versus mild). It has been shown that low HDL and high triglycerides level measured before or during hospitalization were strong predictors of severe COVID-19. Researchers indicate that the lipid profile should be considered a sensitive marker of inflammation and should be measured in COVID-19 patients [19]. An interesting study by Yıldırım and Kaya found that a plasma atherogenic index (AIP) greater than 0.6285 was a predictor of in-hospital mortality in patients with COVID-19 and an early biomarker of severe disease [20].
The opposite results were obtained by Petrilli et al. who assessed the impact of dyslipidemia on the risk of hospitalization and the severe course of COVID-19 in 5279 patients. It has been shown that the occurrence of dyslipidemia was not associated with an increased risk of hospitalization (OR = 0.93; 95% CI: 0.75–1.2; p = 0.51) or an increased risk of mortality in COVID-19 (OR = 0.98; 95% CI: 0.82–1.17; p = 0.79) [21]. It was then confirmed by Chang et al. in a retrospective study of 211 patients with mild COVID-19 symptoms. They found that dyslipidemia was not associated with an increased risk of progression to severe COVID-19 (OR = 1.203; 95% CI: 0.010–148.987; p = 0.940) [22]. Similarly, Simonnet et al. found no relationship between dyslipidemia and the course of COVID-19 (OR = 0.68; 95% CI: 0.24–1.97; p = 0.48) [23]. The impact of overweight/obesity as well as dyslipidemia on the risk of starting artificial ventilation was assessed in 124 patients. There was a significant relationship between weight gain (assessed using body mass index; BMI) and the risk of worsening the course of COVID-19, but no such relationship was found for dyslipidemia (OR = 0.68; 95% CI: 0.24–1.97; p = 0.48) [23]. The discrepancies between the results of the above studies may be due to several reasons. First, the criteria for dyslipidemia diagnosis were different between studies (a huge difference in the incidence of dyslipidemia between studies conducted in the United States and China was observed) [17]. Secondly, it was found that in the course of SARS-CoV-2 infection, there are rapid changes in lipid metabolism, which makes it difficult to interprete whether dyslipidemia occurred before or during COVID-19 [17, 24]. Thirdly, based on the results of the above studies, it can be assumed that perhaps dyslipidemia in its own does not increase the risk of the severe course of COVID-19, but serves as a component of diseases that increase such risk, such as obesity or type 2 diabetes, thus may affect the overall prognosis. Especially, diabetes significantly increases the risk of severe course and death from COVID-19. However, a systematic review by Choi et al. showed that probably not dyslipidemia itself, but CVD caused by it, worsen the prognosis in COVID-19 [17]. To sum up, the relationship between the prevalence of dyslipidemia and COVID-19 requires further studies.
A meta-analysis of 13 studies by Moazzami et al. assessed metabolic risk factors for severe COVID-19. It has been shown that the incidence of obesity and diabetes in patients with COVID-19 was 29% (95% CI: 14–47%) and 22% (95% CI: 12–33%), respectively [25]. A study by Caussy et al. showed that the incidence of obesity depended on the course of COVID-19. Using the standardized prevalence index, the authors found that obesity (BMI ≥ 30 kg / m2) was more common in patients with severe and critical COVID-19 compared to the general population (standardized prevalence ratio: PR = 1.35; 95% CI: 1.08–1.66 and PR = 1.89; 95% CI: 1.33–2.53) [26]. A meta-analysis of 20 studies by Faghir-Gangi et al. which included 5515 patients with COVID-19, showed that type 2 diabetes was present in 14% (95% CI: 11–17%) [27]. The presence of both obesity and type 2 diabetes worsened the prognosis of COVID-19 patients. In a meta-analysis of 75 studies by Popkin et al. the impact of obesity on the prognosis of COVID-19 patients was assessed. Obesity has been shown to increase the risk of hospitalization for COVID-19 by 113% (OR = 2.13; 95% CI: 1.74–2.60; p < 0.0001), the risk of being admitted to the intensive care unit by 74% (OR = 1.74; 95% CI: 1.46–2.08; p < 0.0001), a 66% risk of the need for artificial ventilation (OR = 1.66; 95% CI: 1.38–1.99; p < 0.0001) and a 48% risk of death from COVID-19 (OR = 1.48; 95% CI: 1.22–1.80; p < 0.001) [28]. The relationship between diabetes and COVID-19 severity and mortality was assessed in a meta-analysis of 33 studies by Kumar et al. It was shown that diabetes significantly increased the risk of severe COVID-19 (OR = 2.75; 95% CI: 2.09–3.62; p < 0.01) and increased the risk of death from COVID-19 (OR = 1.90; 95% CI: 1.37–2.64; p < 0.01) [29]. Dyslipidemia frequently occurs in obese or diabetic patients. In a study by Kaur and Aeri involving 150 obese patients, it was shown that dyslipidemia occurred in 78% patients [30]. On the other hand, a study by Li et al. which involved 9285 patients with type 2 diabetes showed that dyslipidemia occurred in 59.3% of them [31].
In general, at the moment it is not known whether dyslipidemia increases the risk of severe course and death from COVID-19. However, dyslipidemia frequently occurs in obese and/or type 2 diabetic patients and requires optimal therapy. On the other hand, both obesity and type 2 diabetes are common in COVID-19 patients and significantly worsen their prognosis. As a result, an exact relationship between the incidence of dyslipidemia and the prognosis of COVID-19 patients requires further studies.
Regardless of the above inconsistent results of observational studies on the influence of dyslipidemia on the course of COVID-19, it has been shown that lipid metabolism is disturbed in the course of this disease [24]. Moreover, it was found that the use of statins improved the prognosis of patients with COVID-19 [32].
Influence of COVID-19 on lipid metabolism
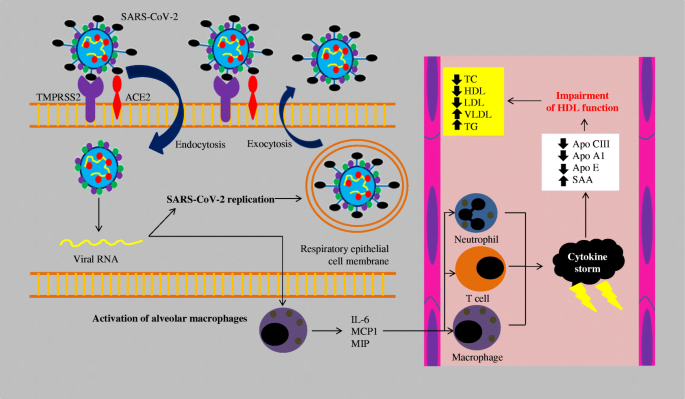
In the course of COVID-19, a temporary disturbance of lipid metabolism was observed, mainly caused by impairment of HDL function (Fig. 1) [33]. The SARS-CoV-2 coronavirus binds to ACE2 via the spike protein (S), which allows entry into the cell. The penetration of SARS-CoV-2 into the lung tissue leads to the activation of alveolar macrophages, which in turn leads to the release of inflammatory mediators, such as: interleukin 6 (IL6), monocyte chemoattractant protein-1 (MCP1) and macrophage inflammatory protein (MIP). These proteins attract further macrophages, neutrophils, and T lymphocytes. The activation of immune system cells leads to the development of uncontrolled inflammation, the so-called cytokine storm and dysregulation of the immune system, as well as the accumulation of eicosanoids, such as: prostaglandin E2 (PGE2), thromboxane B2 (TXB2), leukotriene B4 (LTB4) and lipoxin A4 (LXA4). The uncontrolled inflammation leads to the impairment of HDL lipoprotein function by reducing the concentration of apolipoprotein AI (ApoA-I), apolipoprotein E (ApoE) and increasing the concentration of serum amyloid A (SAA). These changes reduce the anti-inflammatory, antioxidant, and immunomodulatory properties of HDL lipoproteins. Oxidized HDL and LDL lipoproteins (oxLDL and oxHDL) are potent activators of the oxidized LDL scavenge receptor (LOX-1), causing further inflammation and tissue damage. The extracellular portion of serum-soluble LOX-1 (sLOX-1) further stimulates the interaction between oxidized lipids and circulating macrophages, releasing pro-inflammatory cytokines such as IL-6, interleukin 10 (IL-10) and tumor necrosis factor alpha (TNF-α). Impaired function of the enzyme paraoxonase 1 (PON1) located on the surface of HDL lipoprotein and an excessive inflammatory response leads to further lipid oxidation. Increased percentage of oxidized oxLDL and oxHDL lipoproteins leads to the impairment of cholesterol re-transport characterized by insufficient interaction of ApoA-I with the ATP-binding cassette transporter (ABCA1) on macrophages and decreased esterification of cholesterol by lecithin cholesterol acyltransferase (LCAT). The pathophysiological effect of this is reduced return of cholesterol esters to the liver immediately after interaction with hepatic SR-B1 or indirectly after transfer to LDL by cholesterol ester transfer protein (CETP) and uptake by hepatic LDL receptors (LDL-R). Low concentrations of ApoE and apolipoprotein C-III (ApoC-III) on HDL reduce the activity of lipoprotein lipase (LPL), which in turn leads to the accumulation of very low-density lipoproteins (VLDL) and triglycerides. It is also worth mentioning that oxidized phospholipids in LDL lipoproteins are recognized as danger-associated molecular patterns (DAMPs), which leads to inflamosome stimulation and impaired vascular endothelial cell function and atherosclerosis progression. The effects of the interaction of oxLDL and LOX-1 (accumulation of oxLDL inside the cells) also contribute to the accelerated atherosclerosis progression [33].
Lipid disorders induced by SARS-CoV-2 infection [33]. SARS-CoV-2 - severe acute respiratory syndrome coronavirus 2; TMPRSS2 - transmembrane serine protease 2; ACE2 - angiotensin-converting enzyme 2; RNA - ribonucleic acid; IL-6 – interleukin 6; MCP1 - monocyte chemoattractant protein-1; MIP - macrophage inflammatory proteins; Apo CIII - apolipoprotein C-III; Apo A1 – apolipoprotein A1; Apo E – apolipoprotein E; SAA - serum amyloid A; TC – total cholesterol; HDL – high-density lipoprotein; LDL – low-density lipoprotein; VLDL – very-low-density lipoprotein; TG – trigliceryde
The characteristics of lipid metabolism disorders in patients with COVID-19 has been partially confirmed in observational studies (Tab. 1). However, caution should be exercised regarding the results of observations indicating a hypolipidemia initiation in patients with COVID-19 and its impact on the prognosis of this disease. In the commentary to the study by Wei et al. [35] a number of limitations of this type of observation were indicated. It was found that a single assessment of the lipid profile (on admission to the hospital) did not predict the influence of lipid concentration fluctuations on the course of COVID-19. The authors of the comment also state that the adopted criterion of hypolipidemia is not correct. In addition, the effect of lipid-lowering therapy on the results in this study was not included. Thus, the authors of the comment conclude that more analyzes, and studies are needed to establish the relationship between low LDL cholesterol and the course of COVID-19 [39].
Moreover, in the study by Sorokin et al. describing a clinical case of a patient with COVID-19, it was shown that the concentrations of individual serum lipids changed temporarily depending on the disease duration (Fig. 2) [33]. Similar results regarding the level of LDL were obtained by Fan et al. in a study involving 17 patients with COVID-19 [40]. In a study by Li et al. the relationship between changes in serum lipid levels and the prognosis of patients with COVID-19 was assessed. The study included 424 patients with a severe course of COVID-19 (34 survivors and 390 non-survivors). It was shown that during hospitalization LDL, total cholesterol, HDL and ApoA1 showed an increasing trend in survivors but showed a downward trend in non-survivors. Moreover, the serum concentrations of HDL and ApoA1 were inversely correlated with C-reactive protein (CRP), length of hospital stay of survivors, and disease severity scores. Patients with high ratios of CRP/ HDL (> 77.39) or CRP / ApoA1 (> 72.37) had statistically significantly higher mortality rates during hospitalization. The researchers conclude that during severe COVID-19, HDL and ApoA1 concentrations are dramatically decreased in non-survivors. Moreover, high CRP/HDL ratio is significantly associated with an increased mortality and poor prognosis [24]. In the study by Caterino et al. the lipidomic profile and the profile of proinflammatory cytokines and alarmins in COVID-19 patients were analyzed. A significant role of adipose tissue has been demonstrated (in the pathogenesis of lipid disorders observed in patients with COVID-19 [41]. It is worth mentioning that there are ideas addressing the potential role of COVID-19 in the pathogenesis of diabetes. The risk of permanent damage to pancreatic β-cells by SARS-CoV-2 has been suggested. However, this concept requires further research [42].
Lipid profile during COVID-19 – adapted from a paper by Sorokin et al. [33]
Thus, during COVID-19 there are numerous changes in the lipid profile which correlate with the severity of the course of this disease. It should be emphasized that the observed relationship between the severity of COVID-19 and the decrease in lipid levels may not be the cause of poor prognosis, but a consequence of acute inflammatory disease. The pathophysiology of lipid metabolism disorders in patients with COVID-19 has not been fully understood so far. It seems that an important factor disturbing HDL function is the increase serum SAA concentration under the influence of cytokine storm. It has been described that inflammation alters hepatic apolipoprotein gene expression and promotes binding of the pro - inflammatory SAA which, in turn, displaces and decreases ApoA1 concentrations in HDL [43]. Moreover, it was shown that the concentration of SAA in the serum positively correlated with the severity of the course of COVID-19 (p < 0.01) [44].
It is worth mentioning that the lipid disorders observed in patients with COVID-19 are similar to those observed in the course of other infections and inflammatory diseases [45]. In summary, in the course of COVID-19, lipid disorders (very often a decrease in total cholesterol, HDL and LDL, and an increase in triglycerides) caused by inflammation may occur.
Statins and COVID-19: the results form the clinical studies
The effect of statin use on the severity and prognosis of COVID-19 has been the subject of several meta-analyses (Tab. 2.). A study by Lee et al. involving 10,448 COVID-19 patients investigated the effect of statin administration on COVID-19 mortality. Moreover, the effect of statins on the risk of death was compared with a retrospective cohort of patients with pneumonia. Researchers showed a significant decrease in hazard ratio (HR) associated with the use of statins (HR = 0.637; 95% CI: 0.425–0.953; p = 0.0283). Moreover, when comparing the HR between COVID-19 patients and the retrospective cohort of hospitalized pneumonia patients, the use of statins showed similar benefits. Thus, the use of statins correlated significantly with lower mortality in patients with COVID-19, consistent with the findings in patients with pneumonia [59].
Overall, the results of the metaanalysis remain inconsistent when assessing the effect of statins on the improvement of prognosis in COVID-19 patients. Perhaps the conflicting results are an effect of confounding factors such as age, gender, comorbidities, polypharmacy, genetic predisposition, environmental factors, lifestyle, etc. An important factor that could explain divergent metaanalysis results could be the difference in the type of statin used. This possibility was highlighted in a study by Rossi et al. which showed that administration of simvastatin and atorvastatin reduced mortality in COVID-19 patients, whereas those treated with pravastatin and rosuvastatin did not show such a difference [60]. A study by Cariou et al. indicates that the effect of statins may depend on the cardiovascular burden (stage, severity of the underlying disease and comorbidity) of COVID-19 patients [61]. Interpretation of the results of the presented metaanalysis should be exercised with caution, as this type of research is burdened with errors [62]. Moreover, there is a discussion on the methodology used in some meta-analyzes [63, 64]. Future studies should provide more information on the possible benefits of statin treatment in COVID-19 patients. It is known, however, that statin therapy should not be discontinued in patients with COVID-19 [65].
The causal relationship between statin use and the prognosis of COVID-19 patients can only be confirmed by the results of randomized controlled clinical trials (RCTs). A comprehensive review of the literature by Talasaz et al. summarized the ongoing RCTs on statin use (especially atorvastatin and rosuvastatin) in treatment of COVID-19. Moreover, the authors indicate that the role of OMEGA-3 fatty acid, fibrates, and niacin in the treatment of COVID-19 is also under investigation [66]. It should be emphasized that statins will not replace other drugs used in the treatment of COVID-19 patients. Statins can complement therapy in some patients.
It appears that the use of statins in patients with COVID-19 may also contribute to the reduction of the risk of lipid disorders observed in the long-term follow-up of patients infected with other SARS coronaviruses [67].
Statins and COVID-19: mechanism
The beneficial effects of statin administration in COVID-19 patients have been observed in some studies due to their pleiotropic mechanism of action. Several mechanisms by which statins have a positive effect on the prognosis of COVID-19 patients have been described [68]. There are direct and indirect mechanisms of statin action in relation to SARS-CoV-2 infection [69].
Direct effect of statins
Cell membrane cholesterol is involved in the penetration of SARS-CoV-2 into the cell. As shown in Fig. 1, the process of SARS-CoV-2 penetration into the host cell requires the presence of the ACE2 protein. The presence of lipid rafts, which are subdomains of the cell membrane and containing significant amounts of cholesterol, has been shown to play an important role in promoting viral infections [7]. Lipid rafts are important in the interaction between the SARS-CoV-2 spike protein (S) and ACE2 and in virus endocytosis into cell [7]. The important role of ACE2 in coronavirus infections has already been demonstrated in the case of SARS-CoV-1. It was shown that ACE2-knockout mice infected with SARS-CoV-1 had significantly lower viral replication, S protein RNA levels and lung damage compared to wild-type mice with normal ACE2 expression [70]. Another confirmation of the significant role of lipid rafts in SARS-CoV-2 infection are the results of the study by Lu et al. The authors showed that ACE2 was co-localized in lipid rafts biomarkers, i.e., caveolin-1 and monosialotetrahexosyl ganglioside (GM1). Moreover, ACE2 was found to have been transferred to an environment other than the raft after reducing the cholesterol content of the lipid raft [71]. Interestingly, the penetration of SARS-CoV-2 into the cell may be mediated by proteins such as caveolins, clathrins, and dynamin located in lipid rafts [72]. The significant role of cholesterol in coronavirus infection was additionally supported by the study of the effect of cholesterol depletion in SARS-CoV infection, which resulted in a significant reduction of viral mRNA inside the cell [71]. By reducing endogenous cholesterol synthesis, statins reduce its amount in lipid rafts, which may limit the penetration of SARS-CoV-2 into the host cell [7]. Another direct important mechanism of action of statins is direct inhibition of SARS-CoV-2 replication. In silico studies it was shown that pitavastatin, rosuvastatin, lovastatin, and fluvastatin showed high affinity to the main protease SARS-CoV-2 (Mpro), which is involved in the regulation of replication and transcription of this virus [73]. The most important polymerase responsible for the RNA replication of the SARS-CoV-2 coronavirus is RNA-dependent RNA polymerase (RdRp). In a study by Baby et al. it was shown that pitavastatin strongly binds to the active site of this enzyme, as demonstrated by the simulation of molecular dynamics. The authors indicate that the demonstrated mechanism may be used in the treatment of SARS-CoV-2 infection [74].
Thus, statins may exert a direct inhibitory effect on SARS-CoV-2 penetration into the cell and its multiplication. These mechanisms require confirmation in vitro.
Indirect effect of statins
Statins reduce the overexpression of pro-inflammatory cytokines (may reduce the severity of the cytokine storm accompanying COVID-19). The most important cytokine involved in the cytokine storm in COVID-19 is IL-6. It has been shown that the level of IL-6 positively correlates with the severity of COVID-19 [75]. High levels of IL-6 in serum may contribute to the development of a cytokine storm, in addition to the cytokine storm, and the macrophage activation syndrome (MAS), a severe inflammation caused by activated macrophages, manifested by fever, hyperferritinemia, hypofibrinogenemia, coagulopathy and cytopenia [76]. Previous studies have shown that statins reduce IL-6 levels. A meta-analysis of 19 randomized clinical trials by Bonsu et al. including 6214 patients with heart failure, showed that statins are able to lower serum levels of both IL-6 and CRP, with a clear predominance of lipophilic statins, eg. atorvastatin, simvastatin and pitavastatin [77]. The mechanism of action of statins responsible for lowering the level of IL-6 is complex and consists of inhibiting the toll-like receptor 4 (TLR-4) and thus the pro-inflammatory action of the nuclear factor kappa B (NFκB) [69]. In mouse cells, atorvastatin was shown to reduce TLR-4 gene expression [78]. The effect of statins in reducing the risk of MAS is so far poorly understood and requires further research [69].
It is known that the vascular endothelium is damaged during COVID-19, therefore an interesting effect of statins is their effect on the vascular endothelium. It has been shown that statins protect the vascular endothelium from free radicals [69], reduce the pro-inflammatory activity of the NOD-like receptor family, pyrin domain containing 3 inflammosome (NLRP3) [79] and maximize the regenerative capacity of the vascular endothelium by increasing the level of human endothelial progenitor cells (EPCs) [80].
Another thing worth mentioning are the anticoagulant properties of statins. Thromboembolic complications are relatively common in COVID-19 patients. A multicentre retrospective study found the overall thrombotic complication rate associated with COVID-19 is 9.5% (95% CI: 6.8–12.8) [81]. Previous studies have shown that the use of statins (especially atorvastatin and rosuvastatin) reduced the risk of recurrent pulmonary embolism, which is one of the most serious thromboembolic diseases [82]. The explanation for the above beneficial effects of statins is their influence on the level of plasminogen activator inhibitor-1 (PAI-1). A meta-analysis of 16 randomized controlled trials conducted by Sahebkar et al. showed that statins (especially atorvastatin) significantly decreased the level of plasminogen activator inhibitor-1 (PAI-1) in the serum, thus increasing the degradation of fibrin clots by the enzyme plasmin [83]. It has also been shown that statins have an anticoagulant effect by reducing the plasma level of von Willebrand factor antigen [84].
The anti-fibrotic effect of statins seems to be very interesting from the point of view of complications of SARS-CoV-2 infection (especially in the long-COVID-19 syndrome). In a study by Li et al. which included 107 patients with COVID-19, it was shown that after 3–6 months after recovery, some of them may develop pulmonary fibrosis [85]. In an experimental study using mice and human lung fibroblasts/myofibroblasts, the effect of atorvastatin on the processes of fibrosis was assessed. In mice, administration of atorvastatin has been shown to reduce the number of fibrosis and collagen accumulation in an interstitial tissue, as well as reduced the levels of alpha-smooth muscle actin (α-SMA), lysyl oxidase-like protein 2 (LOXL2) and p-Src. In vitro studies have shown a reduction in α-SMA and fibronectin levels by limiting the action of transforming growth factor beta (TGF-β) [86]. It has also been suggested that statins inhibit the epithelial-mesenchymal transition (EMT) by attenuating TGF-β signaling known to be associated with post-infectious pulmonary fibrosis, causing remodeling and deposition of connective tissue among fibroblasts and epithelial cells [87, 88]. Statins also increase fibroblast apoptosis [89].
It is also worth mentioning that statins, by increasing the level of HDL lipoproteins, on average can have antiviral effects in this way. It has been shown that HDL lipoproteins can bind lipopolysaccharide as well as lipoteichoic acid [90, 91]. Moreover, HDL binding to lipopolysaccharide protects animals from the toxicity of this endotoxin [92]. Moreover, HDL can block the penetration of some viruses into cells, reducing their infection and multiplication in various tissues [93]. Moreover, HDL lipoproteins are characterized by antioxidant, anticoagulant, immunomodulating and anti-inflammatory properties, and also participate in the regeneration of the vascular endothelium [94]. The observed reduction in HDL lipoprotein levels by 40–70% in inflammatory diseases, including COVID-19, may further worsen the course of the disease [94].
A very interesting indirect antiviral mechanism of statin action is the effect of these drugs on arachidonic acid levels. A review of the literature by Hoxha concluded that arachidonic acid deficiency may increase the risk of developing COVID-19 [95]. A review of the literature by Das even points to the potential role of arachidonic acid in the prevention and treatment of COVID-19 [96]. A study by Risé et al. showed that statins significantly increase the concentration of arachidonic acid in the plasma in patients with hypercholesterolemia [97]. In an in vitro study by Goc et al., the effect of polyunsaturated ω-3 fatty acids (including arachidonic acid) on penetration of SARS-CoV-2 into the cell interior was assessed. These acids have been shown to interfere with the binding of SARS-CoV-2 to ACE2 on the cell surface [98]. Thus, statins may make it difficult for SARS-CoV-2 to infect cells by increasing arachidonic acid synthesis.
Thus, statins have many effects, both direct and indirect, that can improve the prognosis of COVID-19 patients. The mechanisms of beneficial effects of statin administration in COVID-19 patients are presented in Fig. 3.
Clinical recommendations for patients with familial hypercholesterolemia
Considering that patients with familial hypercholesterolemia (heterozygous and homozygous) may be at increased risk of a severe course of COVID-19, Banach et al. developed brief recommendations for the treatment of this group of patients during the COVID-19 pandemic. The authors of the recommendation indicate that there is a number of scientific evidence indicating the potential beneficial effect of statins in reducing the severity of COVID-19. Patients with familial hypercholesterolemia should especially respect the therapeutic principles and social distancing, as their cardiovascular risk is much higher than in the general population. Treatment control should be done using telemedicine services, and medications should be prescribed for a longer period of time. In the event of a new diagnosis of familial hypercholesterolemia, the patient should be immediately referred to a specialist center for telephone advice and treatment. The use of statins in patients with COVID-19 is generally safe. All patients who require regular lipid apheresis should have access to this procedure [99]. emphasizing the need for intensive lipid-lowering therapy in that particular group of patients. As indicated by the guidelines of the European Society of Cardiology (ESC), the use of statins in the course of COVID-19 is safe in the majority of patients and may improve the prognosis of patients. Therefore, stopping statin therapy is not recommended [100]. It is woth emphasizing, that based on multiple data available on the role of statin therapy in COVID-19 patients in the just released Polish guidelines on the diagnosis and therapy of lipid disorders, for the first time in the world, the experts made an attempt at recommendations on the use of statins in patients with COVID-19 (Tab 3.).
Discussion
The issues of the impact of lipid disorders on the prognosis in COVID-19, the impact of COVID-19 on lipid metabolism and the role of statins in improving the prognosis of COVID-19 patients presented in this article are consistent with the authors’ observations and other articles describing these issues [102, 103]. It seems that effective lipid-lowering treatment, not only with statins but also with other drugs, improves the prognosis of COVID-19 patients. A study by Israel et al. assessed the effect of various medications taken by patients with COVID-19 on the course of the disease. It was shown that the use of rosuvastatin and ezetimibe significantly reduced the risk of hospitalization due to COVID-19 (OR = 0.673, 95% CI: 0.596–0.758, p < 0.001 and OR = 0.488, 95% CI: 0.377–0.622), p < 0.001, respectively) [104]. Barkas et al. suggest that proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, through their immunomodulatory properties, may reduce the risk of serious complications of COVID-19, such as acute respiratory distress syndrome and cytokine release syndrome [105]. It is indicated that PCSK9 reduces the expression of interferon β (INF-β) [106]. Therefore, the use of PCSK9 inhibitors, especially in patients with hypercholesterolemia, should reduce this adverse effect and improve the antiviral efficiency of the organism related to INF-β [107]. Overall, it has been suggested that lipid-lowering treatments, other than statins therapy, may also possibly reduce the severity of COVID-19, but this issue requires further research.
We strongly believe that optimal lipid-lowering therapy may improve the prognosis of patients with COVID-19. So, following the other recommendations we agree that strict control of lipid parameters in blood is of crucial importance especially during COVID-19 pandemic.
Compared to the previous ones, our article comprehensively presents the two-way relationship between lipid disorders and COVID-19. We critically discussed the cited research results and indicated the need to exercise caution when interpreting them. We have presented the latest meta-analyzes and clinical recommendations for the use of statins during the COVID-19 pandemic.
Conclusions
Lipid disorders may increase the risk of a severe course of COVID-19, but on the other hand SARS-CoV-2 infection may itself cause lipid disorders in some patients, mainly impairing the function of HDL lipoproteins (which is obviously less important from the lclinical point of view). The use of statins may reduce the risk of severe caurse of COVID-19 and reduce the risk of death in these patients [108]. Statins, through their pleiotropic mechanism of action, can reduce the penetration of SARS-CoV-2 into the host cell and reduce the risk of complications from cytokine storm [109]. Patients with familial hypercholesterolaemia, and those being at high and very high CVD risk, may be at risk of the severe course of COVID-19. Therefore, lipid-lowering treatment should be particularly monitored in this group of patients.
A number of RCTs is currently underway to investigate the effect of statin use on COVID-19 score. More and more pathophysiological mechanisms linking lipidological problems with COVID-19 are under investigation, which will be finally answer all the existing questions on mechanisms and outcomes.
Availability of data and materials
Not applicable.
Abbreviations
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- COVID-19:
-
Coronavirus disease 2019
- RR:
-
Relative risk
- CVD:
-
Cardiovascular disease
- OR:
-
Odds ratio
- ACE2:
-
Angiotensin converting enzyme 2
- SR-B1:
-
Scavenger receptor, class B type 1
- LDL:
-
Low density lipoprotein
- HDL:
-
High density lipoprotein
- AIP:
-
Plasma atherogenic index
- BMI:
-
Body mass index
- PR:
-
Prevalence ratio
- IL-6:
-
Interleukin 6
- MCP-1:
-
Monocyte chemoattractant protein-1
- MIP:
-
macrophage inflammatory proteins
- PGE2:
-
Prostaglandin E2
- TXB2:
-
Thromboxane B2
- LTB4:
-
Leukotriene B4
- LXA4:
-
Lipoxin A4
- ApoA1:
-
Apolipoprotein A1
- ApoE:
-
Apolipoprtoein E
- SAA:
-
Serum amyloid A
- oxLDL:
-
Oxidized low density lipoprotein
- oxHDL:
-
Oxidized high density lipoprotein
- LOX1:
-
Oxidized LDL scavenge receptor
- sLOX1:
-
Soluble oxidized LDL scavenge receptor 1
- IL-10:
-
Interleukin 10
- TNFα:
-
Tumor necrosis factor alpha
- PON1:
-
Paraoxonase 1
- ABCA1:
-
ATP-binding cassette transporter 1
- LCAT:
-
Lecithin–cholesterol acyltransferase
- CETP:
-
Cholesteryl ester transfer protein
- LDL-R:
-
Low density lipoprotein receptor
- ApoCIII:
-
Apolipoprotein CIII
- LPL:
-
Lipoprotein lipase
- VLDL:
-
Very-low density lipoprotein
- CRP:
-
C-reactive protein
- HR:
-
Hazard ratio
- RCTs:
-
Randomized controlled trials
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 1
- RNA:
-
Ribonucleic acid
- GM1:
-
Monosialotetrahexosylganglioside 1
- mRNA:
-
Messenger ribonucleic acid
- Mpro:
-
Protease SARS-CoV-2
- RdRp:
-
RNA-dependent RNA polymerase
- MAS:
-
Macrophage activation syndrome
- TLR-4:
-
Toll-like receptor 4
- NFκB:
-
Nuclear factor kappa B
- NLRP3:
-
NOD-like receptor family, pyrin domain containing 3 inflammosome
- EPCs:
-
Endothelial progenitor cells
- PAI-I:
-
Plasminogen activator inhibitor-1
- α-SMA:
-
Alpha-smooth muscle actin
- LOXL2:
-
Lysyl oxidase-like protein 2
- TGF-β:
-
Transforming growth factor beta
- EMT:
-
Epithelial-mesenchymal transition
- ESC:
-
EUROPEAN Society of Cardiology
- PCSK9:
-
Proprotein convertase subtilisin/kexin type 9
- INF-β:
-
Interferon beta
References
Dömling A, Gao L. Chemistry and biology of SARS-CoV-2. Chem. 2020;6(6):1283–95. https://doi.org/10.1016/j.chempr.2020.04.023.
Dos Santos W. Natural history of COVID-19 and current knowledge on treatment therapeutic options. Biomed Pharmacother. 2020;129:110493. https://doi.org/10.1016/j.biopha.2020.110493.
Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo P, Cuapio A, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Res Sq. 2021:rs.3.rs-266574.
Iqbal FM, Lam K, Sounderajah V, Clarke JM, Ashrafian H, Darzi A. Characteristics and predictors of acute and chronic post-COVID syndrome: a systematic review and meta-analysis. EClinicalMedicine. 2021;36:100899. https://doi.org/10.1016/j.eclinm.2021.100899.
Shi C, Wang L, Ye J, Gu Z, Wang S, Xia J, et al. Predictors of mortality in patients with coronavirus disease 2019: a systematic review and meta-analysis. BMC Infect Dis. 2021;21(1):663. https://doi.org/10.1186/s12879-021-06369-0.
Bae S, Kim SR, Kim M-N, Shim WJ, Park S-M. Impact of cardiovascular disease and risk factors on fatal outcomes in patients with COVID-19 according to age: a systematic review and meta-analysis. Heart. 2021;107(5):373–80. https://doi.org/10.1136/heartjnl-2020-317901.
Radenkovic D, Chawla S, Pirro M, Sahebkar A, Banach M. Cholesterol in relation to COVID-19: should we care about it? J Clin Med. 2020;9(6):1909. https://doi.org/10.3390/jcm9061909.
Pirillo A, Casula M, Olmastroni E, Norata GD, Catapano AL. Global epidemiology of dyslipidaemias. Nat Rev Cardiol. 2021. https://doi.org/10.1038/s41569-021-00541-4.
Kočar E, Režen T, Rozman D. Cholesterol, lipoproteins, and COVID-19: basic concepts and clinical applications. Biochim Biophys Acta Mol Cell Biol Lipids. 1866;2021(2):158849. https://doi.org/10.1016/j.bbalip.2020.158849.
Glende J, Schwegmann-Wessels C, Al-Falah M, et al. Importance of cholesterol-rich membrane microdomains in the interaction of the S protein of SARS-coronavirus with the cellular receptor angiotensin-converting enzyme 2. Virology. 2008;381(2):215–21. https://doi.org/10.1016/j.virol.2008.08.026.
Toelzer C, Gupta K, Yadav SKN, Borucu U, Davidson AD, Kavanagh Williamson M, et al. Free fatty acid binding pocket in the locked structure of SARS-CoV-2 spike protein. Science. 2020;370(6517):725–30. https://doi.org/10.1126/science.abd3255.
Shoemark DK, Colenso CK, Toelzer C, Gupta K, Sessions RB, Davidson AD, et al. Molecular simulations suggest vitamins, retinoids and steroids as ligands of the free fatty acid pocket of the SARS-CoV-2 spike protein. Angew Chem Int Ed Engl. 2021;60(13):7098–110. https://doi.org/10.1002/anie.202015639.
Wei C, Wan L, Zhang Y, et al. Cholesterol metabolism — Impact for SARS-CoV-2 infection prognosis, entry, and antiviral therapies. medRxiv. 2020. https://doi.org/10.1101/2020.04.16.20068528.
Atmosudigdo IS, Pranata R, Lim MA, Henrina J, Yonas E, Vania R, et al. Dyslipidemia increases the risk of severe COVID-19: a systematic review, meta-analysis, and meta-regression. J Clin Exp Hepatol. 2021. https://doi.org/10.1016/j.jceh.2021.01.007.
Hariyanto TI, Kurniawan A. Dyslipidemia is associated with severe coronavirus disease 2019 (COVID-19) infection. Diabetes Metab Syndr. 2020;14(5):1463–5. https://doi.org/10.1016/j.dsx.2020.07.054.
Santos CS, Morales CM, Álvarez ED, Castro CÁ, Robles AL, Sandoval TP. Determinants of COVID-19 disease severity in patients with underlying rheumatic disease. Clin Rheumatol. 2020;39(9):2789–96. https://doi.org/10.1007/s10067-020-05301-2.
Choi GJ, Kim HM, Kang H. The potential role of dyslipidemia in COVID-19 severity: an umbrella review of systematic reviews. J Lipid Atheroscler. 2020;9(3):435–48. https://doi.org/10.12997/jla.2020.9.3.435.
Liu Y, Pan Y, Yin Y, Chen W, Li X. Association of dyslipidemia with the severity and mortality of coronavirus disease 2019 (COVID-19): a meta-analysis. Virol J. 2021;18(1):157. https://doi.org/10.1186/s12985-021-01604-1.
Masana L, Correig E, Ibarretxe D, et al. Low HDL and high triglycerides predict COVID-19 severity. Sci Rep. 2021;11(1):7217. https://doi.org/10.1038/s41598-021-86747-5.
Yıldırım ÖT, Kaya Ş. The atherogenic index of plasma as a predictor of mortality in patients with COVID-19. Heart Lung. 2021;50(2):329–33. https://doi.org/10.1016/j.hrtlng.2021.01.016.
Petrilli CM, Jones SA, Yang J, Rajagopalan H, O’Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in new York City: prospective cohort study. BMJ. 2020;369:1966. https://doi.org/10.1136/bmj.m1966.
Chang MC, Park Y-K, Kim B-O, Park D. Risk factors for disease progression in COVID-19 patients. BMC Infect Dis. 2020;20(1):445. https://doi.org/10.1186/s12879-020-05144-x.
Simonnet A, Chetboun M, Poissy J. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020;28(7):1195–9. https://doi.org/10.1002/oby.22831.
Li Y, Zhang Y, Lu R, Dai M, Shen M, Zhang J, et al. Lipid metabolism changes in patients with severe COVID-19. Clin Chim Acta. 2021;517:66–73. https://doi.org/10.1016/j.cca.2021.02.011.
Moazzami B, Chaichian S, Kasaeian A, Djalalinia S, Akhlaghdoust M, Eslami M, et al. Metabolic risk factors and risk of Covid-19: a systematic review and meta-analysis. PLoS One. 2020;15(12):e0243600. https://doi.org/10.1371/journal.pone.0243600.
Caussy C, Pattou F, Wallet F, Simon C, Chalopin S, Telliam C, et al. Prevalence of obesity among adult inpatients with COVID-19 in France. Lancet Diabetes Endocrinol. 2020;8(7):562–4. https://doi.org/10.1016/S2213-8587(20)30160-1.
Faghir-Gangi M, Moameri H, Abdolmohamadi N, Nematollahi S. The prevalence of type 2 diabetes in patients with COVID-19: a systematic review and meta-analysis. Clin Diabetol. 2020;9(5):271–8. https://doi.org/10.5603/DK.2020.0041.
Popkin BM, Du S, Green WD, et al. Individuals with obesity and COVID-19: a global perspective on the epidemiology and biological relationships. Obes Rev. 2020;21(11):e13128. https://doi.org/10.1111/obr.13128.
Kumar A, Arora A, Shrama P, et al. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab Syndr. 2020;14(4):535–45. https://doi.org/10.1016/j.dsx.2020.04.044.
Kaur H, Aeri BT. Assessing the prevalence of dyslipidemia in apparently healthy urban obese adults residing in South Delhi. India J Gizi Pangan. 2020;15(2):63–70. https://doi.org/10.25182/jgp.2020.15.2.63-70.
Li Y, Zhao L, Yu D, Ding G. The prevalence and risk factors of dyslipidemia in different diabetic progression stages among middle-aged and elderly populations in China. PLoS One. 2018;13(10):e0205709. https://doi.org/10.1371/journal.pone.0205709.
Vahedian-Azimi A, Mohammadi SM, Beni FH, Banach M, Guest PC, Jamialahmadi T, et al. Improved COVID-19 ICU admission and mortality outcomes following treatment with statins: a systematic review and meta-analysis. Arch Med Sci. 2021;17(3):579–95. https://doi.org/10.5114/aoms/132950.
Sorokin AV, Karathanasis SK, Yang Z-H, Freeman L, Kotani K, Remaley AT. COVID-19-associated dyslipidemia: implications for mechanism of impaired resolution and novel therapeutic approaches. FASEB J. 2020;34(8):9843–53. https://doi.org/10.1096/fj.202001451.
Hu X, Chen D, Wu L, He G, Ye W. Declined serum high density lipoprotein cholesterol is associated with the severity of COVID-19 infection. Clin Chim Acta. 2020;510:105–10. https://doi.org/10.1016/j.cca.2020.07.015.
Wei X, Zeng W, Su J, Wan H, Yu X, Cao X, et al. Hypolipidemia is associated with the severity of COVID-19. J Clin Lipidol. 2020;14(3):297–304. https://doi.org/10.1016/j.jacl.2020.04.008.
Wang G, Zhang Q, Zhao X, Dong H, Wu C, Wu F, et al. Low high-density lipoprotein level is correlated with the severity of COVID-19 patients: an observational study. Lipids Health Dis. 2020;19(1):204. https://doi.org/10.1186/s12944-020-01382-9.
Wang D, Li R, Wang J, Jiang Q, Gao C, Yang J, et al. Correlation analysis between disease severity and clinical and biochemical characteristics of 143 cases of COVID-19 in Wuhan. China: a descriptive study BMC Infect Dis. 2020;20(1):519. https://doi.org/10.1186/s12879-020-05242-w.
Zhang Q, Wei Y, Chen M, Wan Q, Chen X. Clinical analysis of risk factors for severe COVID-19 patients with type 2 diabetes. J Diabetes Complicat. 2020;34(10):107666. https://doi.org/10.1016/j.jdiacomp.2020.107666.
Fogacci F, Bogrhi C, Cicero AFG. Misinterpreting data in lipidology in the era of COVID-19. J Clin Lipidol. 2020;14(4):543–4. https://doi.org/10.1016/j.jacl.2020.07.004.
Fan J, Wang H, Ye G, Cao X, Xu X, Tan W, et al. Letter to the editor: low-density lipoprotein is a potential predictor of poor prognosis in patients with coronavirus disease 2019. Metabolism. 2020;107:154243. https://doi.org/10.1016/j.metabol.2020.154243.
Caterino M, Gelzo M, Sol S, Fedele R, Annunziata A, Calabrese C, et al. Dysregulation of lipid metabolism and pathological inflammation in patients with COVID-19. Sci Rep. 2021;11(1):2941. https://doi.org/10.1038/s41598-021-82426-7.
Accili D. Can COVID-19 cause diabetes? Nat Metab. 2021;3(2):123–5. https://doi.org/10.1038/s42255-020-00339-7.
Han CY, Chiba T, Campbell JS, Fausto N, Chaisson M, Orasanu G, et al. Reciprocal and coordinate regulation of serum amyloid a versus apolipoprotein A-I and paraoxonase-1 by inflammation in murine hepatocytes. Arterioscler Thromb Vasc Biol. 2006;26(8):1806–13. https://doi.org/10.1161/01.ATV.0000227472.70734.ad.
Li H, Xiang X, Ren H, Xu L, Zhao L, Chen X, et al. Serum amyloid a is a biomarker of severe coronavirus disease and poor prognosis. J Inf Secur. 2020;80(6):646–55. https://doi.org/10.1016/j.jinf.2020.03.035.
Feingold KR, Grunfeld C. The effect of inflammation and infection on lipids and lipoproteins. In: Endotext. South Dartmouth: MDText.com, Inc.; 2000–2019.
Kow CS, Hasan SS. Meta-analysis of effect of statins in patients with COVID-19. Am J Cardiol. 2020;134:153–5. https://doi.org/10.1016/j.amjcard.2020.08.004.
Scheen AJ. Statins and clinical outcomes with COVID-19: meta-analyses of observational studies. Diabetes Metab. 2020;47(6):101220. https://doi.org/10.1016/j.diabet.2020.101220.
Onorato D, Pucci M, Carpene G, Henry BM, Sanchis-Gomar F, Lippi G. Protective effects of statins administration in European and north American patients infected with COVID-19: a meta-analysis. Semin Thromb Hemost. 2021;47(4):392–9. https://doi.org/10.1055/s-0040-1722307.
Pal R, Banerjee M, Yadav U, Bhattacharjee S. Statin use and clinical outcomes in patients with COVID-19: an updated systematic review and meta-analysis. Postgrad Med J. 2021. https://doi.org/10.1136/postgradmedj-2020-139172.
Chow R, Im J, Chiu N, et al. The protective association between statins use and adverse outcomes among COVID-19 patients: a systematic review and meta-analysis. medRxiv. https://doi.org/10.1101/2021.02.08.21251070.
Wu K-S, Lin P-C, Chen Y-S, Pan T-C, Tang P-L. The use of statins was associated with reduced COVID-19 mortality: a systematic review and meta-analysis. Ann Med. 2021;53(1):874–84. https://doi.org/10.1080/07853890.2021.1933165.
Permana H, Huang I, Purwiga A, Kusumawardhani NY, Sihite TA, Martanto E, et al. In-hospital use of statins is associated with a reduced risk of mortality in coronavirus-2019 (COVID-19): systematic review and meta-analysis. Pharmacol Rep. 2021;73(3):1–12. https://doi.org/10.1007/s43440-021-00233-3.
Yetmar ZA, Chesdachai S, Kashour T, et al. Prior statin use and risk of mortality and severe disease from coronavirus disease 2019: a systematic review and meta-analysis. Open Forum Infect Dis. 2021;8:ofab284.
Hariyanto TI, Kurniawan A. Statin and outcomes of coronavirus disease 2019 (COVID-19): a systematic review, meta-analysis, and meta-regression. Nutr Metab Cardiovasc Dis. 2021;31(6):1662–70. https://doi.org/10.1016/j.numecd.2021.02.020.
Zein AFMZ, Sulistiyana CS, Khasanah U, Wibowo A, Lim MA, Pranata R. Statin and mortality in COVID-19: a systematic review and meta-analysis of pooled adjusted effect estimates from propensity-matched cohorts. Postgrad Med J. 2021. https://doi.org/10.1136/postgradmedj-2021-140409.
Diaz-Arocutipa C, Melgar-Talavera B, Alvarado-Yarasca Á, Saravia-Bartra MM, Cazorla P, Belzusarri I, et al. Statins reduce mortality in patients with COVID-19: an updated meta-analysis of 147 824 patients. Int J Infect Dis. 2021;110:374–81. https://doi.org/10.1016/j.ijid.2021.08.004.
Kollias A, Kyriakoulis KG, Kyriakoulis IG, Nitsotolis T, Poulakou G, Stergiou GS, et al. Statin use and mortality in COVID-19 patients: updated systematic review and meta-analysis. Atherosclerosis. 2021;330:114–21. https://doi.org/10.1016/j.atherosclerosis.2021.06.911.
Kow CS, Hasan SS. The association between the use of statins and clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. Am J Cardiovasc Drugs. 2021:1–15. https://doi.org/10.1007/s40256-021-00490-w.
Lee H-Y, Ahn J, Park J, et al. Beneficial effect of statins in COVID-19-related outcomes-brief report: a national population-based cohort study. Arterioscler Thromb Vasc Biol. 2021;41:175–82.
Rossi R, Talarico M, Coppi F, Boriani G. Protective role of statins in COVID 19 patients: importance of pharmacokinetic characteristics rather than intensity of action. Intern Emerg Med. 2020;15(8):1573–6. https://doi.org/10.1007/s11739-020-02504-y.
Cariou B, Goronflot T, Rimbert A, Boullu S, le May C, Moulin P, et al. Routine use of statins and increased COVID-19 related mortality in inpatients with type 2 diabetes: results from the CORONADO study. Diabetes Metab. 2021;47(2):101202. https://doi.org/10.1016/j.diabet.2020.10.001.
Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323(7304):101–5. https://doi.org/10.1136/bmj.323.7304.101.
Tandaju JR, Ii W, Barati-Boldaji R, Raeisi-Dehkordi H. Meta-analysis of statin and outcomes of coronavirus disease 2019 (COVID-19): reconsideration is needed. Nutr Metab Cardiovasc Dis. 2021;31(9):2737–9. https://doi.org/10.1016/j.numecd.2021.06.009.
Hariyanto TI, Kurniawan A. Authors’ response: meta-analysis of statin and outcomes of coronavirus disease 2019 (COVID-19). Nutr Metab Cardiovasc Dis. 2021;31(9):2740–2. https://doi.org/10.1016/j.numecd.2021.06.008.
Iqbal Z, Ho JH, Adam S, France M, Syed A, Neely D, et al. Managing hyperlipidaemia in patients with COVID-19 and during its pandemic: an expert panel position statement from HEART UK. Atherosclerosis. 2020;313:126–36. https://doi.org/10.1016/j.atherosclerosis.2020.09.008.
Talasaz AH, Sadeghipour P, Aghakouchakzadeh M, et al. Lipid-modulating agents for prevention or treatment of COVID-19 in randomized trials. medRxiv. 2021:2021.05.03.21256468.
Wu Q, Zhou L, Sun X, Yan Z, Hu C, Wu J, et al. Altered lipid metabolism in recovered SARS patients twelve years after infection. Sci Rep. 2017;7(1):9110. https://doi.org/10.1038/s41598-017-09536-z.
Ferrara F, Vitiello A. The advantages of drug treatment with statins in patients with SARS-CoV-2 infection. Wien Klin Wochenschr. 2021;133(17-18):1–8. https://doi.org/10.1007/s00508-021-01845-8.
Pawlos A, Niedzielski M, Gorzelak-Pabiś P, Broncel M, Woźniak E. COVID-19: direct and indirect mechanisms of statins. Int J Mol Sci. 2021;22(8):4177. https://doi.org/10.3390/ijms22084177.
Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11(8):875–9. https://doi.org/10.1038/nm1267.
Lu Y, Liu DX, Tam JP. Lipid rafts are involved in SARS-CoV entry into vero E6 cells. Biochem. Biophys. Res Commun. 2008;369:344–9.
Baglivo M, Baronio M, Natalini G, Beccari T, Chiurazzi P, Fulcheri E, et al. Natural small molecules as inhibitors of coronavirus lipid-dependent attachment to host cells: a possible strategy for reducing SARS-COV-2 infectivity? Acta Biomed. 2020;91(1):161–4. https://doi.org/10.23750/abm.v91i1.9402.
Reiner Ž, Hatamipour M, Banach M, Pirro M, Al-Rasadi K, Jamialahmadi T, et al. Statins and the COVID-19 main protease: in silico evidence on direct interaction. Arch Med Sci. 2020;16(3):490–6. https://doi.org/10.5114/aoms.2020.94655.
Baby K, Maity S, Mehta CH, Suresh A, Nayak UY, Nayak Y. Targeting SARS-CoV-2 RNA-dependent RNA polymerase: an in silico drug repurposing for COVID-19. F1000Res. 2020;9:1166.
Coomes EA, Haghbayan H. Interleukin-6 in Covid-19: a systematic review and meta-analysis. Rev Med Virol. 2020;30(6):1–9. https://doi.org/10.1002/rmv.2141.
Henderson LA, Cron RQ. Macrophage activation syndrome and secondary Hemophagocytic Lymphohistiocytosis in childhood inflammatory disorders: diagnosis and management. Pediatr Drugs. 2020;22(1):29–44. https://doi.org/10.1007/s40272-019-00367-1.
Bonsu KO, Reidpath DD, Kadirvelu A. Effects of statin treatment on inflammation and cardiac function in heart failure: an adjusted indirect comparison Meta-analysis of randomized trials. Cardiovasc Ther. 2015;33(6):338–46. https://doi.org/10.1111/1755-5922.12150.
Chansrichavala P, Chantharaksri U, Sritara P, Chaiyaroj SC. Atorvastatin attenuates TLR4-mediated NF-κB activation in a MyD88-dependent pathway. Asian Pac J Allergy Immunol. 2009;27:49–57.
Wang S, Xie X, Lei T, Zhang K, Lai B, Zhang Z, et al. Statins attenuate activation of the NLRP3 Inflammasome by oxidized LDL or TNF<i>α</i> in vascular endothelial cells through a PXR-dependent mechanism. Mol Pharmacol. 2017;92(3):256–64. https://doi.org/10.1124/mol.116.108100.
Oikonomou E, Siasos G, Zaromitidou M, Hatzis G, Mourouzis K, Chrysohoou C, et al. Atorvastatin treatment improves endothelial function through endothelial progenitor cells mobilization in ischemic heart failure patients. Atherosclerosis. 2015;238(2):159–64. https://doi.org/10.1016/j.atherosclerosis.2014.12.014.
Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489–500. https://doi.org/10.1182/blood.2020006520.
Biere-Rafi S, Hutten BA, Squizzato A, Ageno W, Souverein PC, de Boer A, et al. Statin treatment and the risk of recurrent pulmonary embolism. Eur Heart J. 2013;34(24):1800–6. https://doi.org/10.1093/eurheartj/eht046.
Sahebkar A, Catena C, Ray KK, Vallejo-Vaz AJ, Reiner Ž, Sechi LA, et al. Impact of statin therapy on plasma levels of plasminogen activator inhibitor-1. A systematic review and meta-analysis of randomised controlled trials. Thromb Haemost. 2016;116(1):162–71. https://doi.org/10.1160/TH15-10-0770.
Sahebkar A, Serban C, Ursoniu S, Mikhailidis DP, Undas A, Lip GYH, et al. The impact of statin therapy on plasma levels of von Willebrand factor antigen: systematic review and meta-analysis of randomised placebo-controlled trials. Thromb Haemost. 2016;115(03):520–32. https://doi.org/10.1160/th15-08-0620.
Li G, Du L, Cao X, et al. Follow-up study on serum cholesterol profiles and potential sequelae in recovered COVID-19 patients. BMC Infect Dis. 2021;21(1):299. https://doi.org/10.1186/s12879-021-05984-1.
Yildirim M, Kayalar O, Atahan E, Oztay F. Anti-fibrotic effect of atorvastatin on the lung fibroblasts and myofibroblasts. Eur Resp J. 2018;52:PA991.
Saito A, Horie M, Nagase T. TGF-β signaling in lung health and disease. Int J Mol Sci. 2018;19(8):2460. https://doi.org/10.3390/ijms19082460.
Yang T, Chen M, Sun T. Simvastatin attenuates TGF-β1-induced epithelial-mesenchymal transition in human alveolar epithelial cells. Cell Physiol Biochem. 2013;31(6):863–74. https://doi.org/10.1159/000350104.
Saewong S, Thammasitboon K, Wattanaroonwong N. Simvastatin induces apoptosis and disruption of the actin cytoskeleton in human dental pulp cells and periodontal ligament fibroblasts. Arch Oral Biol. 2013;58(8):964–74. https://doi.org/10.1016/j.archoralbio.2013.03.002.
Lee R-P, Lin N-T, Chao Y-FC, Lin C-C, Harn H-J, Chen H-I. High-density lipoprotein prevents organ damage in endotoxemia. Res Nurs Health. 2007;30(3):250–60. https://doi.org/10.1002/nur.20187.
Grunfeld C, Marshall M, Shigenaga JK, Moser AH, Tobias P, Feingold KR. Lipoproteins inhibit macrophage activation by lipoteichoic acid. J Lipid Res. 1999;40(2):245–52. https://doi.org/10.1016/S0022-2275(20)33363-0.
Guo L, Ai J, Zheng Z, Howatt DA, Daugherty A, Huang B, et al. High density lipoprotein protects against polymicrobe-induced sepsis in mice. J Biol Chem. 2013;288(25):17947–53. https://doi.org/10.1074/jbc.M112.442699.
Feingold KR, Grunfeld C. Lipids: a key player in the battle between the host and microorganisms. J Lipid Res. 2012;53(12):2487–9. https://doi.org/10.1194/jlr.E033407.
Stasi A, Franzin R, Fiorentino M, Squiccimarro E, Castellano G, Gesualdo L. Multifaced roles of HDL in sepsis and SARS-CoV-2 infection: renal implications. Int J Mol Sci. 2021;22(11):5980. https://doi.org/10.3390/ijms22115980.
Hoxha M. What about COVID-19 and arachidonic acid pathway? Eur J Clin Pharmacol. 2020;76(11):1501–4. https://doi.org/10.1007/s00228-020-02941-w.
Das UN. Bioactive lipids-based therapeutic approach to COVID-19 and other similar infections. Arch Med Sci. 2021; in press. https://doi.org/10.5114/aoms/135703.
Risé P, Pazzucconi F, Sirtori CR, Galli C. Statins enhance arachidonic acid synthesis in hypercholesterolemic patients. Nutr Metab Cardiovasc Dis. 2001;11(2):88–94.
Goc A, Niedzwiecki A, Rath M. Polyunsaturated ω-3 fatty acids inhibit ACE2-controlled SARS-CoV-2 binding and cellular entry. Sci Rep. 2021;11(1):5207. https://doi.org/10.1038/s41598-021-84850-1.
Banach M, Penson P, Fras Z, Vrablik M, Pella D, Reiner Ž, et al. Brief recommendations on the management of adult patients with familial hypercholesterolemia during the COVID-19 pandemic. Pharmacol Res. 2020;158:104891. https://doi.org/10.1016/j.phrs.2020.104891.
Andreini D, Arbelo E, Barbato E, et al. ESC Guidance for the Diagnosis and Management of CV Disease during the COVID-19 Pandemic. https://www.escardio.org/Education/COVID-19-and-Cardiology/ESC-COVID-19-Guidance
Banach M, Burchardt P, Chlebus K, et al. PoLA/CFPiP/PCS/PSLD/PSD/PSH guidelines on the diagnosis and tehrapy of lipid disorders in Poland. Arch Med Sci. 2021;17(6). https://doi.org/10.5114/aoms/141941.
Minz MM, Bansal M, Kasliwal RR. Statins and SARS-CoV-2 disease: current concepts and possible benefits. Diabetes Metab Syndr. 2020;14(6):2063–7. https://doi.org/10.1016/j.dsx.2020.10.021.
Vahedian-Azimi A, Mohammadi SM, Banach M, et al. Improved COVID-19 Outcomes following Statin Therapy: An Updated Systematic Review and Meta-analysis. Biomed Res Int. 2021;2021:1901772. https://doi.org/10.1155/2021/1901772.
Israel A, Schäffer AA, Cicurel A, et al. Identification of drugs associated with reduced severity of COVID-19: A case-control study in a large population. medRxiv. 2021:2020.10.13.20211953.
Barkas A, Milionis H, Anastasiou G, Liberopoulos S. Statins and PCSK9 inhibitors: what is their role in coronavirus disease 2019? Med Hypotheses. 2021;146:110452. https://doi.org/10.1016/j.mehy.2020.110452.
Li Z, Liu Q. Proprotein convertase subtilisin/kexin type 9 inhibits interferon β expression through interacting with ATF-2. FEBS Lett. 2018;592(13):2323–33. https://doi.org/10.1002/1873-3468.13152.
Vuorio A, Kovanen PT. PCSK9 inhibitors for COVID-19: an opportunity to enhance the antiviral action of interferon in patients with hypercholesterolaemia. J Intern Med. 2021;289(5):749–51. https://doi.org/10.1111/joim.13210.
Katsiki N, Banach M, Mikhailidis DP. More good news on statins and COVID-19. Am J Cardiol. 2021;138:127–8.
Bhaskar S, Sinha A, Banach M, Mittoo S, Weissert R, Kass JS, et al. Cytokine storm in COVID-19-immunopathological mechanisms, clinical considerations, and therapeutic approaches: the REPROGRAM Consortium Position Paper. Front Immunol. 2020;11:1648. https://doi.org/10.3389/fimmu.2020.01648.
Acknowledgments
Not applicable.
Declaration of interests
MB has received research grant(s)/support from Amgen, Mylan, Sanofi, and Valeant, and has served as a consultant for Akcea, Amgen, Daiichi-Sankyo, Esperion, Freia Pharmaceuticals, Herbapol, Kogen, KRKA, Mylan, Novartis, Novo-Nordisk, Polfarmex, Polpharma, Resverlogix, Sanofi-Regeneron, Servier, Teva, and Zentiva.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MB - idea and project of work; SS and JL - searching databases; SS and JL wrote the manuscript; SS designed figures and tables; JL - linguistic improvement of the text; MB - final approval of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The author declared that he has no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Surma, S., Banach, M. & Lewek, J. COVID-19 and lipids. The role of lipid disorders and statin use in the prognosis of patients with SARS-CoV-2 infection. Lipids Health Dis 20, 141 (2021). https://doi.org/10.1186/s12944-021-01563-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-021-01563-0